
Chemistry, 12.02.2021 08:40 asjessehills2000
The monovalent salt concentration (the predominant solute in the blood cell) for a sample of red blood cells is 0.13 moles/liter. If one of these red blood cells were placed in pure water (at around room temperature, 300 K), and the cell comes to hydrostatic equilibrium with the water, what is the osmotic pressure of the cell (assuming it doesn't burst)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
The monovalent salt concentration (the predominant solute in the blood cell) for a sample of red blo...
Questions

Mathematics, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30



Biology, 26.01.2021 04:30

French, 26.01.2021 04:30


Mathematics, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30

Spanish, 26.01.2021 04:30

Medicine, 26.01.2021 04:30



Mathematics, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30


Mathematics, 26.01.2021 04:30

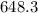 KPa
KPa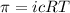
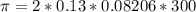
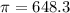 KPa
KPa

