
Chemistry, 19.09.2019 18:30 woodfordmaliky
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(aq)+3h2(g) (deltah)= -725.0 kj
2. hcl(> hcl(aq) (deltah)= -74.8kj 3. h2(g)+cl2(g) --> 2hcl(g) (deltah)=-1845.0kj 4. mcl3(s) --> mcl3(aq) (deltah)= -476.0kj use the information above to determine the enthalpy of the following reaction.
2m(s)+3cl2(g) > 2mcl3(s) (deltah) = kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

You know the right answer?
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
Questions

Mathematics, 23.04.2021 04:30

Mathematics, 23.04.2021 04:30

Chemistry, 23.04.2021 04:30

Mathematics, 23.04.2021 04:30




Mathematics, 23.04.2021 04:30




English, 23.04.2021 04:30


English, 23.04.2021 04:30



Social Studies, 23.04.2021 04:30



French, 23.04.2021 04:30

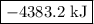
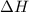 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.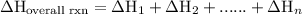
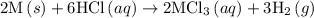 ...… (1)
...… (1)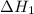 is -725 kJ.
is -725 kJ.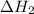 .
.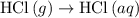 ...... (2)
...... (2) 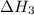 .
.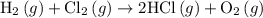 …… (3)
…… (3)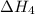 .
.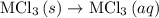 …… (4)
…… (4)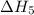 .
.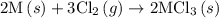 …… (5)
…… (5)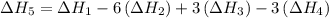 ...... (7)
...... (7) 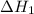 , -74.8 kJ for
, -74.8 kJ for 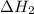 and -1845 kJ for
and -1845 kJ for 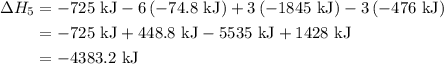
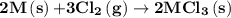 is -4383.2 kJ.
is -4383.2 kJ.

