
Chemistry, 12.02.2021 05:20 nancye2008
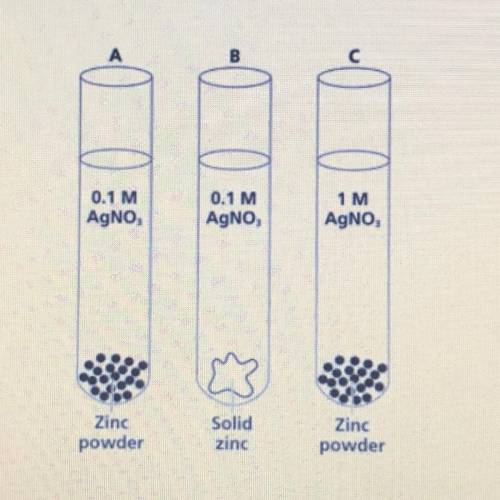
Which of the following test tubes
A) would have the fastest rate of reaction? Defend your answer.
B) would have the slowest rate of reaction? Defend your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
Which of the following test tubes
A) would have the fastest rate of reaction? Defend your answer.
Questions

Advanced Placement (AP), 25.05.2021 22:00



History, 25.05.2021 22:00



Mathematics, 25.05.2021 22:00

Mathematics, 25.05.2021 22:00

Mathematics, 25.05.2021 22:00

Chemistry, 25.05.2021 22:00

Mathematics, 25.05.2021 22:00


Health, 25.05.2021 22:00





English, 25.05.2021 22:00


Mathematics, 25.05.2021 22:00



