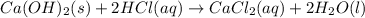Chemistry, 11.02.2021 23:20 babygirl123468
A sample of 7.34 g of solid calcium hydroxide is added to 34.5 mL of 0.380 M Aqueous hydrochloric acid. Write the balanced chemical equation for the reaction. Physical states are optional.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
A sample of 7.34 g of solid calcium hydroxide is added to 34.5 mL of 0.380 M Aqueous hydrochloric ac...
Questions

Social Studies, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30




Mathematics, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30



English, 05.11.2020 20:30


Mathematics, 05.11.2020 20:30

Mathematics, 05.11.2020 20:30


Mathematics, 05.11.2020 20:30



History, 05.11.2020 20:30