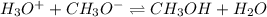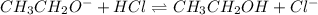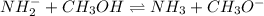Chemistry, 11.02.2021 21:40 laurielaparr2930
Name each of the following species for the following acid-base reactions. (The equilibrium lies to the right in each case, i. e., the product side is favored. If the species is an ion, include the word "ion" in the name. Use systematic names such as "methanol" instead of archaic names like "methyl alcohol" or "wood alcohol".)
(a) H3O+ (hydronium ion) + CH3O- (methoxide ion) <--> *reverse reaction arrow*
acid:?
base:?
conjugate acid:?
conjugate base:?
(b) CH3CH2O- (ethoxide ion) + HCl (hydrogen chloride) <--> *reverse reaction arrow*
acid:?
base:?
conjugate acid:?
conjugate base:?
(c) NH2- (amide ion) + CH3OH (methanol) <--> *reverse reaction arrow*
acid:?
base:?
conjugate acid:?
conjugate base:?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

You know the right answer?
Name each of the following species for the following acid-base reactions. (The equilibrium lies to t...
Questions


Mathematics, 25.04.2020 19:47





Mathematics, 25.04.2020 19:47



Mathematics, 25.04.2020 19:47


Mathematics, 25.04.2020 19:47


Chemistry, 25.04.2020 19:47



Mathematics, 25.04.2020 19:47


Social Studies, 25.04.2020 19:47