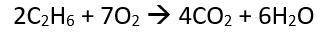Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
2 Questions:
What is the maximum amount of CO2 that can be produced when 35.0g C2H6 and 75.0g O2 re...
Questions



English, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Biology, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00



Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00

Engineering, 04.10.2021 14:00


Mathematics, 04.10.2021 14:00

Mathematics, 04.10.2021 14:00


Social Studies, 04.10.2021 14:00