
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 25. g of ethane is mixed with 122. g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions



World Languages, 07.07.2021 08:00


Mathematics, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00


Physics, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00


Business, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00

Social Studies, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00

Mathematics, 07.07.2021 08:00



Physics, 07.07.2021 08:10

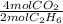 = 1.67 mol CO₂
= 1.67 mol CO₂

