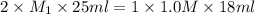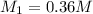Chemistry, 20.01.2020 01:31 rtryf57rfgth
A25-ml solution of h2so4 is completely neutralized by 18 ml of 1.0m naoh. what is the concentration of the h2so4?
h2so4(aq) +2naoh(aq) _ na2so4(aq) + 2h2o(l)
a.0.72m
b.0.009m
c.0.50m
d.0.36m

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1


Chemistry, 23.06.2019 13:30
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3
You know the right answer?
A25-ml solution of h2so4 is completely neutralized by 18 ml of 1.0m naoh. what is the concentration...
Questions










Social Studies, 07.08.2019 05:10




Social Studies, 07.08.2019 05:10

Social Studies, 07.08.2019 05:10

Social Studies, 07.08.2019 05:10


English, 07.08.2019 05:10


Mathematics, 07.08.2019 05:10

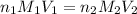
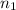 = basicity of an acid = 2
= basicity of an acid = 2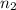 = acidity of a base = 1
= acidity of a base = 1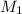 = concentration of
= concentration of 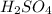 = ?
= ?
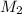 = concentration of NaOH = 1.0 M
= concentration of NaOH = 1.0 M
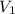 = volume of
= volume of 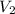 = volume of NaOH = 18 ml
= volume of NaOH = 18 ml