
Chemistry, 11.02.2021 14:00 abraham1366
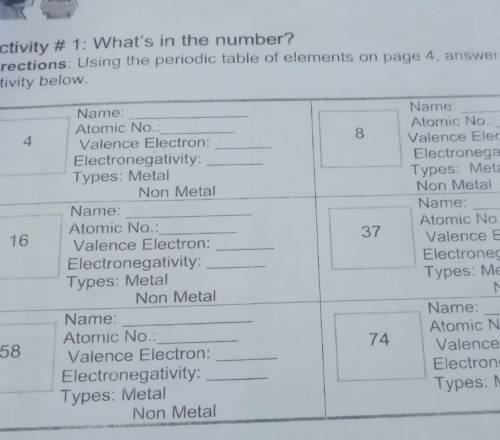
Activity # 1: What's in the number?
Directions: Using the periodic table of elements on page 4, answer the given
activity below
4
8
16
37
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
Name:
Atomic No.:
Valence Electron:
Electronegativity:
Types: Metal
Non Metal
58
74 pa hellp plsss

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
Activity # 1: What's in the number?
Directions: Using the periodic table of elements on page 4, ans...
Questions


History, 04.07.2019 16:00




English, 04.07.2019 16:00


Mathematics, 04.07.2019 16:00






History, 04.07.2019 16:00


History, 04.07.2019 16:00

History, 04.07.2019 16:00



Mathematics, 04.07.2019 16:00



