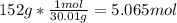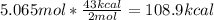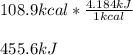Chemistry, 11.02.2021 14:00 santiagoagilg
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are produced in the following chemical reaction, 43
kcal of heat energy is "absorbed."
N2(g) + O2(g) → 2 NO(g), AH = +43 kcal
How much heat (in kJ) is exchanged when 152 g of NO(g) is produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are...
Questions


Advanced Placement (AP), 15.10.2020 02:01


Mathematics, 15.10.2020 02:01



Mathematics, 15.10.2020 02:01



Mathematics, 15.10.2020 02:01



Mathematics, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01

Social Studies, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01


Mathematics, 15.10.2020 02:01

Mathematics, 15.10.2020 02:01