
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δh∘=−484kj
how much pv work is done in kilojoules for the reaction of 3.20 mol of h2 with 1.60 mol of o2 at atmospheric pressure if the volume change is −22.6l?
express your answer using three significant figures

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δ...
Questions

Mathematics, 17.09.2019 06:30

Social Studies, 17.09.2019 06:30

Biology, 17.09.2019 06:30


History, 17.09.2019 06:30

English, 17.09.2019 06:30


Mathematics, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30

Biology, 17.09.2019 06:30

History, 17.09.2019 06:30



Social Studies, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30

Physics, 17.09.2019 06:30



English, 17.09.2019 06:30

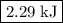 .
.
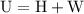
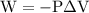
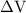 is the change in the volume in liter.
is the change in the volume in liter.
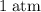 .
.
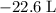 .
.
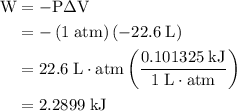
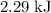 .
.


