
Question 4 (2 points)
Potassium chlorate decomposes into potassium chloride and oxygen gas according to
the following equation:
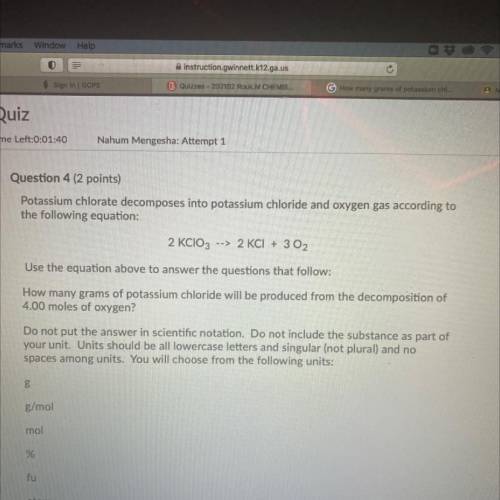
2 KClO3 --> 2 KCl + 3 O2
Use the equation above to answer the questions that follow:
How many grams of potassium chloride will be produced from the decomposition of
4.00 moles of oxygen?
Do not put the answer in scientific notation. Do not include the substance as part of
your unit. Units should be all lowercase letters and singular (not plural) and no
spaces among units. You will choose from the following units:
В
g/mol
mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1



Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Question 4 (2 points)
Potassium chlorate decomposes into potassium chloride and oxygen gas accordin...
Questions

Computers and Technology, 07.04.2020 00:54

Mathematics, 07.04.2020 00:54



History, 07.04.2020 00:54

Mathematics, 07.04.2020 00:54

History, 07.04.2020 00:54



History, 07.04.2020 00:54

History, 07.04.2020 00:54

Biology, 07.04.2020 00:54

Computers and Technology, 07.04.2020 00:54

Mathematics, 07.04.2020 00:54

Mathematics, 07.04.2020 00:54


Mathematics, 07.04.2020 00:54


Mathematics, 07.04.2020 00:54

Computers and Technology, 07.04.2020 00:54



