
Chemistry, 10.02.2021 22:10 kiannadgarnica
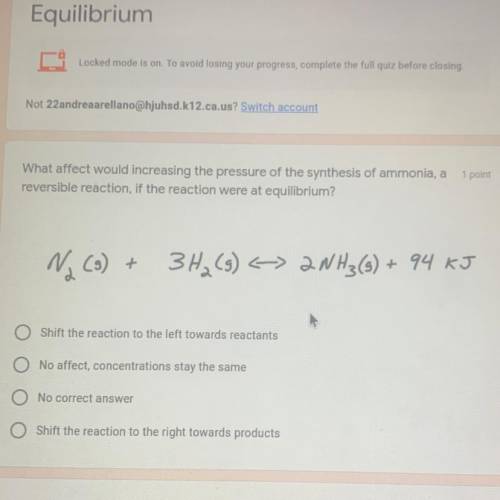
What affect would increasing the pressure of the synthesis of ammonia, a
reversible reaction, if the reaction were at equilibrium?
Na (3) +
3H₂(g) < 2NH3(s) + 94 KJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
What affect would increasing the pressure of the synthesis of ammonia, a
reversible reaction, if th...
Questions










English, 28.05.2020 19:02

Mathematics, 28.05.2020 19:02


Mathematics, 28.05.2020 19:02

Mathematics, 28.05.2020 19:02



Mathematics, 28.05.2020 19:02






