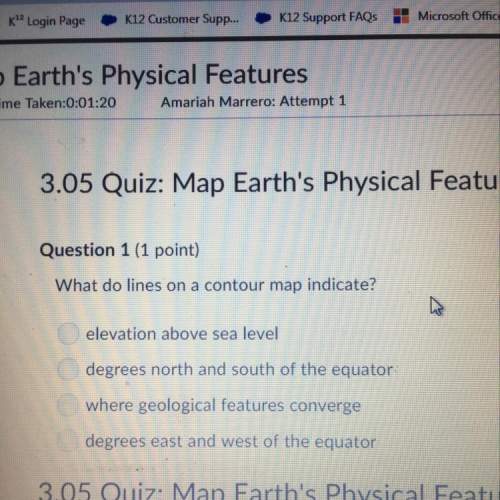
Chemistry, 10.02.2021 16:40 ghari112345
Pls help Would be much appreciated:)
b. A redox reaction occurs in an electrochemical cell, where silver (Ag) is oxidized and nickel (Ni)
is reduced. (5 points)
i. Write the half-reactions for this reaction, indicating the oxidation half-reaction and the
reduction half-reaction. (1 point)
ii. Which metal is the anode, and which is the cathode? (1 point)
iii. Calculate the standard potential (voltage) of the cell. (2 point)
iv. What kind of electrochemical cell is this? Explain your answer. (1 point

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Pls help Would be much appreciated:)
b. A redox reaction occurs in an electrochemical cell, where s...
Questions











Geography, 10.10.2019 00:10


Computers and Technology, 10.10.2019 00:10





Geography, 10.10.2019 00:10

Geography, 10.10.2019 00:10