
Chemistry, 10.02.2021 07:40 angelinararr5783
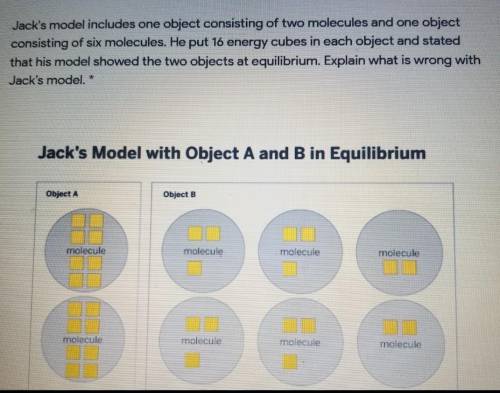
HELP PLZ ITS DUE IN 10 MIN "Jack's model includes one object consisting of two molecules and one object consisting of six molecules. He put 16 energy cubes in each object and stated that his model showed the two objects at equilibrium. Explain what is wrong with Jack's model."
(I know it looks long but basically I just need help with finding what's wrong with the model)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
HELP PLZ ITS DUE IN 10 MIN "Jack's model includes one object consisting of two molecules and one obj...
Questions


Physics, 23.07.2019 11:00




English, 23.07.2019 11:00


English, 23.07.2019 11:00

English, 23.07.2019 11:00

English, 23.07.2019 11:00

History, 23.07.2019 11:00

English, 23.07.2019 11:00

Mathematics, 23.07.2019 11:00


Mathematics, 23.07.2019 11:00



English, 23.07.2019 11:00

Mathematics, 23.07.2019 11:00



