
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganate. They react according to the following equation
KMnO4 + K2C2O4 → K2Mn + 2CO2 + 202
If the reaction has a 53% yield, how many grams of carbon dioxide does it produce?
А
11.79
B
44.09
23.39
2209
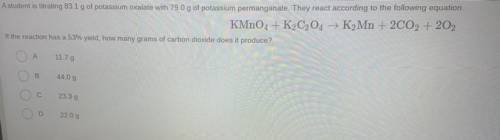

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Question 14
A student is titrating 83.1 g of potassium oxalate with 79.0 g of potassium permanganat...
Questions


Mathematics, 07.11.2021 07:40



Physics, 07.11.2021 07:40

Mathematics, 07.11.2021 07:40





Biology, 07.11.2021 07:40




Mathematics, 07.11.2021 07:50

Social Studies, 07.11.2021 08:00




Mathematics, 07.11.2021 08:00



