
Chemistry, 09.02.2021 15:50 fortwill05
What is the final, balanced equation that is formed by combining these two half reactions? 2 equations: first: upper C u right arrow upper C u superscript 2 plus, plus 2 e superscript minus. Second: upper N upper O subscript 3 superscript minus, plus 2 e superscript minus, plus 2 upper H superscript plus right arrow upper n upper O subscript 2 superscript minus plus upper H subscript 2 upper O.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
You know the right answer?
What is the final, balanced equation that is formed by combining these two half reactions? 2 equatio...
Questions

Mathematics, 11.12.2020 04:00

Social Studies, 11.12.2020 04:00




Mathematics, 11.12.2020 04:00

Mathematics, 11.12.2020 04:00



English, 11.12.2020 04:00



Mathematics, 11.12.2020 04:00




Mathematics, 11.12.2020 04:00


Mathematics, 11.12.2020 04:00


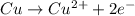 (1)
(1)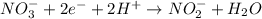 (2)
(2)

