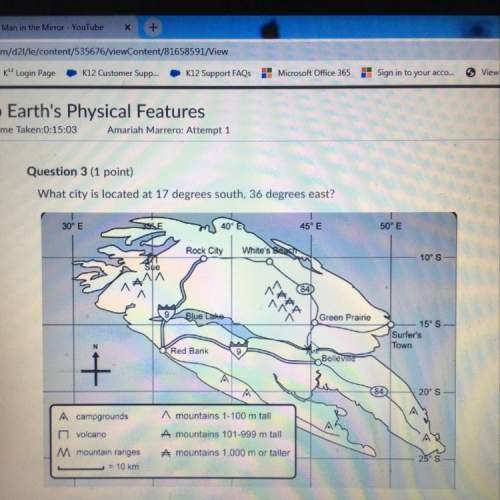
Chemistry, 09.02.2021 03:20 kenzieraerae6771
A glass sphere is filled to full volume with a gas. The pressure of the gas inside the sphere is 30.0 atm, and the temperature is 30.0°C. The sphere is taken outside on a cold day. The temperature of the gas decreases to 10.0°C. What is the new pressure of the gas? Assume that the volume is constant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
A glass sphere is filled to full volume with a gas. The pressure of the gas inside the sphere is 30....
Questions


Biology, 11.05.2021 06:00


English, 11.05.2021 06:00


History, 11.05.2021 06:00


History, 11.05.2021 06:00

English, 11.05.2021 06:00

Social Studies, 11.05.2021 06:00


Chemistry, 11.05.2021 06:00


English, 11.05.2021 06:00





Mathematics, 11.05.2021 06:00

Chemistry, 11.05.2021 06:00