40 POINTS!
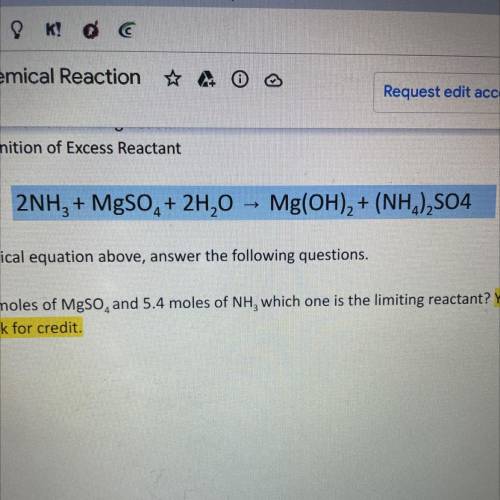
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation ab...

Chemistry, 09.02.2021 01:30 emalvidrez5205
40 POINTS!
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation above answer the following questions. SHOW ALL WORK OR RECEIVE NO CREDIT
A. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting reactant? Show work for credit
B. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of MgSO4 and 5.4 moles of NH3? Show work for credit
C. How many moles of the excess reactant is left over after the reaction has been completed? Show work for credit

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Questions

Business, 05.01.2021 23:00


Health, 05.01.2021 23:00

Chemistry, 05.01.2021 23:00



Spanish, 05.01.2021 23:00

Mathematics, 05.01.2021 23:00



Mathematics, 05.01.2021 23:00

Mathematics, 05.01.2021 23:00

Chemistry, 05.01.2021 23:00


Mathematics, 05.01.2021 23:00

Chemistry, 05.01.2021 23:00

Mathematics, 05.01.2021 23:00


World Languages, 05.01.2021 23:00



