
Chemistry, 09.02.2021 01:00 Josediego55
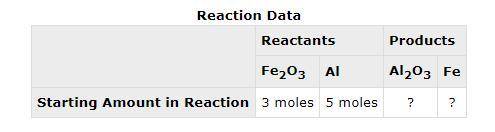
The following data was collected when a reaction was performed experimentally in the laboratory. Determine the maximum amount of Fe that was produced during the experiment. Explain how you determined this amount.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
The following data was collected when a reaction was performed experimentally in the laboratory.
De...
Questions



English, 09.03.2021 22:30




Arts, 09.03.2021 22:30

Mathematics, 09.03.2021 22:30





Mathematics, 09.03.2021 22:30

Mathematics, 09.03.2021 22:30






English, 09.03.2021 22:30



