
Chemistry, 08.02.2021 22:00 carmenala2
A reaction that proceeds by first-order irreversible kinetics is oxidizing chemical A in a wastewater treatment basin with a mean residence time of 1.5 hours. The reaction rate constant is The basin is unbaffled and may be characterized as two completely mixed tanks in series. If the steady-state influent concentration is 30 mg/l, find the effluent concentration. If baffles are placed in the basin so that the basin may be characterized as four completely mixed tanks in series, and the mean residence time remains constant, find the effluent concentration.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

You know the right answer?
A reaction that proceeds by first-order irreversible kinetics is oxidizing chemical A in a wastewate...
Questions


Mathematics, 09.04.2021 18:40



Mathematics, 09.04.2021 18:40



Business, 09.04.2021 18:40

Social Studies, 09.04.2021 18:40

Mathematics, 09.04.2021 18:40

Mathematics, 09.04.2021 18:40

Mathematics, 09.04.2021 18:40

Mathematics, 09.04.2021 18:40


Health, 09.04.2021 18:40





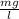
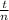 )ⁿ
)ⁿ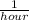 )
)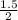 )²
)²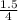 )⁴
)⁴


