Chemistry, 08.02.2021 21:20 jhenifelix
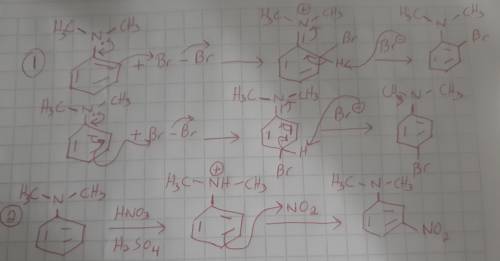
When N, N-Dimethylaniline is treated with bromine, ortho and para products are observed. However, when N, N-Dimethylaniline is treated with a mixture of nitric and sulfuric acid, only the meta product is observed. Explain these results. (Hint: what can happen to the nitrogen atom in the presence of the strong acids

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
When N, N-Dimethylaniline is treated with bromine, ortho and para products are observed. However, wh...
Questions

History, 16.09.2019 22:30


Computers and Technology, 16.09.2019 22:30

Computers and Technology, 16.09.2019 22:30

Computers and Technology, 16.09.2019 22:30



Mathematics, 16.09.2019 22:30

Computers and Technology, 16.09.2019 22:30

Chemistry, 16.09.2019 22:30







Computers and Technology, 16.09.2019 22:30