
Chemistry, 08.02.2021 20:30 SophieStar15
ANSWER ASAP
Which of the following electronic transitions in the oxygen atom will result in light emission?
. A. ni = 1 ⟶ nf = 2
B. ni = 1 ⟶ nf = 3
C. ni = 3 ⟶ nf = 2
D. ni = 2 ⟶ nf = 3
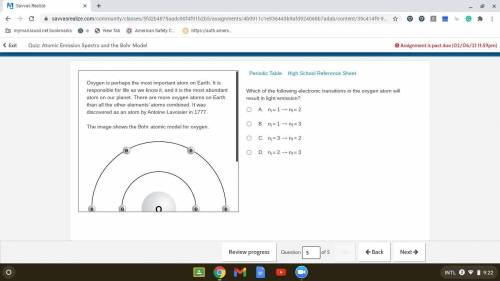
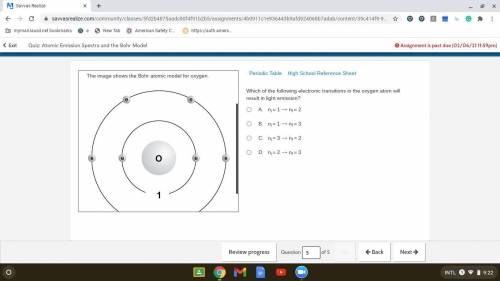

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
ANSWER ASAP
Which of the following electronic transitions in the oxygen atom will result in light e...
Questions


Geography, 19.10.2021 07:50

Mathematics, 19.10.2021 07:50



Biology, 19.10.2021 07:50

Social Studies, 19.10.2021 07:50

Mathematics, 19.10.2021 07:50

Mathematics, 19.10.2021 07:50




Social Studies, 19.10.2021 07:50

Medicine, 19.10.2021 07:50

Mathematics, 19.10.2021 07:50



Mathematics, 19.10.2021 07:50


Mathematics, 19.10.2021 07:50



