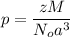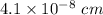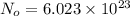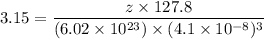Chemistry, 08.02.2021 19:20 SumayahAminaAnsari
A hypothetical AX type of ceramic material is known to have a density of 3.15 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.41 nm. The atomic weights of the A and X elements are 90.5 and 37.3 g/mol, respectively. On the basis of this information, which one of the following crystal structures is possible for this material?
a. Sodium chloride
b. Cesium chloride
c. Zinc blende

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
A hypothetical AX type of ceramic material is known to have a density of 3.15 g/cm3 and a unit cell...
Questions

Mathematics, 08.10.2019 02:00

Mathematics, 08.10.2019 02:00




Mathematics, 08.10.2019 02:00

English, 08.10.2019 02:00

Mathematics, 08.10.2019 02:00

Mathematics, 08.10.2019 02:00




Mathematics, 08.10.2019 02:00

History, 08.10.2019 02:00


Biology, 08.10.2019 02:00

Biology, 08.10.2019 02:00

Arts, 08.10.2019 02:00

Mathematics, 08.10.2019 02:00

English, 08.10.2019 02:00