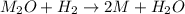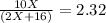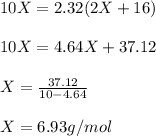Chemistry, 08.02.2021 14:00 zairaefh3200
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal
and one mole of water. 5.00 g of the metallic oxide produces 2.32 g of the metal. What is the metallic
oxide? (Use molar masses)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 14:30
William has eight more nickels than dimes in his pocket for a total of $2.50. which equation could be used to determine the number of x dimes in his pocket?
Answers: 1
You know the right answer?
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal...
Questions



Mathematics, 01.09.2020 20:01


Mathematics, 01.09.2020 20:01

Social Studies, 01.09.2020 20:01



Chemistry, 01.09.2020 20:01

History, 01.09.2020 20:01


Mathematics, 01.09.2020 20:01


Chemistry, 01.09.2020 20:01




Engineering, 01.09.2020 20:01


Biology, 01.09.2020 21:01