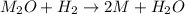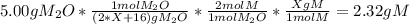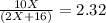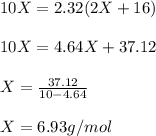Chemistry, 08.02.2021 09:40 madiforkner
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal
and one mole of water. 5.00 g of the metallic oxide produces 2.32 g of the metal. What is the metallic
oxide? (Hint: use the molar masses)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

You know the right answer?
One mole of a metallic oxide reacts with one mole of hydrogen to produce two moles of the pure metal...
Questions

English, 18.10.2019 04:30






SAT, 18.10.2019 04:30


Mathematics, 18.10.2019 04:30

Chemistry, 18.10.2019 04:30

Biology, 18.10.2019 04:30




Mathematics, 18.10.2019 04:30



English, 18.10.2019 04:30

Mathematics, 18.10.2019 04:30

Mathematics, 18.10.2019 04:30