
Chemistry, 08.02.2021 05:00 20jhutchinson
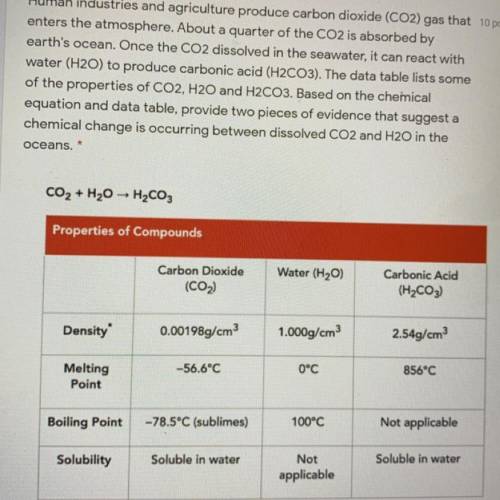
Human industries and agriculture produce carbon dioxide (CO2) gas that
enters the atmosphere. About a quarter of the CO2 is absorbed by
earth's ocean. Once the CO2 dissolved in the seawater, it can react with
water (H20) to produce carbonic acid (H2CO3). The data table lists some
of the properties of CO2, H20 and H2CO3. Based on the chemical
equation and data table, provide two pieces of evidence that suggest a
chemical change is occurring between dissolved CO2 and H2O in the
oceans.
CO2 + H2O → H2CO3
Properties of Compounds
Water (H20)
Carbon Dioxide
(CO2)
Carbonic Acid
(H2CO3)
Density
0.00198g/cm3
1.000g/cm3
2.54g/cm3
0°C
856°C
-56.6°C
Melting
Point
100°C
Not applicable
Boiling Point
-78.5°C (sublimes)
Soluble in water
Solubility
Soluble in water
Not
applicable

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Human industries and agriculture produce carbon dioxide (CO2) gas that
enters the atmosphere. About...
Questions


English, 03.10.2019 13:30

Mathematics, 03.10.2019 13:30





History, 03.10.2019 13:30

Mathematics, 03.10.2019 13:30





History, 03.10.2019 13:30

Spanish, 03.10.2019 13:30

Mathematics, 03.10.2019 13:30


History, 03.10.2019 13:30




