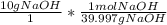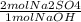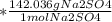Chemistry, 08.02.2021 02:30 ariellake8551
(05.04 MC)
The following reaction shows sodium hydroxide reacting with sulfuric acid.
NaOH + H2SO4 → Na2SO4 + H2O
How many grams of Na2SO4 are produced from 10.0 grams of NaOH?
(Molar mass of Na = 22.989 g/mol, O = 15.999 g/mol, H = 1.008 g/mol, S = 32.065 g/mol) (4 points)
a
17.8 grams
b
19.2 grams
c
35.5 grams
d
38.5 grams
will mark brainliest

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2


Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
(05.04 MC)
The following reaction shows sodium hydroxide reacting with sulfuric acid.
N...
N...
Questions


Mathematics, 04.02.2020 12:47

History, 04.02.2020 12:47

Mathematics, 04.02.2020 12:47

History, 04.02.2020 12:47

Mathematics, 04.02.2020 12:47



Mathematics, 04.02.2020 12:47



Biology, 04.02.2020 12:47


Spanish, 04.02.2020 12:47


Physics, 04.02.2020 12:47


Mathematics, 04.02.2020 12:47

Mathematics, 04.02.2020 12:47