Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
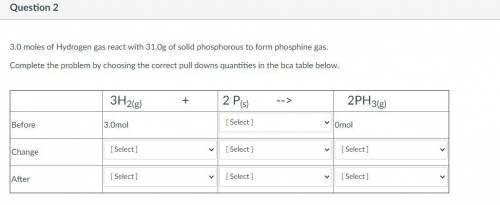
3.0 moles of Hydrogen gas react with 31.0g of solid phosphorous to form phosphine gas.
Complete the...
Questions

Computers and Technology, 04.12.2020 22:10

Biology, 04.12.2020 22:10

Computers and Technology, 04.12.2020 22:10



Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

World Languages, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

English, 04.12.2020 22:10


Mathematics, 04.12.2020 22:10

Mathematics, 04.12.2020 22:10

History, 04.12.2020 22:10


Social Studies, 04.12.2020 22:10