
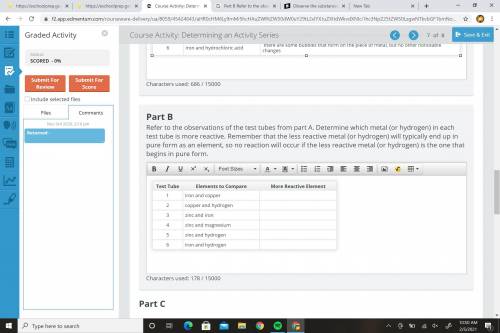
Please help me... Refer to the observations of the test tubes from part A. Determine which metal (or hydrogen) in each test tube is more reactive. Remember that the less reactive metal (or hydrogen) will typically end up in pure form as an element, so no reaction will occur if the less reactive metal (or hydrogen) is the one that begins in pure form.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
Please help me...
Refer to the observations of the test tubes from part A. Determine which metal (...
Questions

Chemistry, 21.07.2021 19:20

Mathematics, 21.07.2021 19:20

Mathematics, 21.07.2021 19:20

Mathematics, 21.07.2021 19:20

English, 21.07.2021 19:20




English, 21.07.2021 19:20


English, 21.07.2021 19:20


English, 21.07.2021 19:20




Mathematics, 21.07.2021 19:20

English, 21.07.2021 19:20

Mathematics, 21.07.2021 19:20

Mathematics, 21.07.2021 19:20



