
Chemistry, 05.02.2021 06:10 qqbear4555
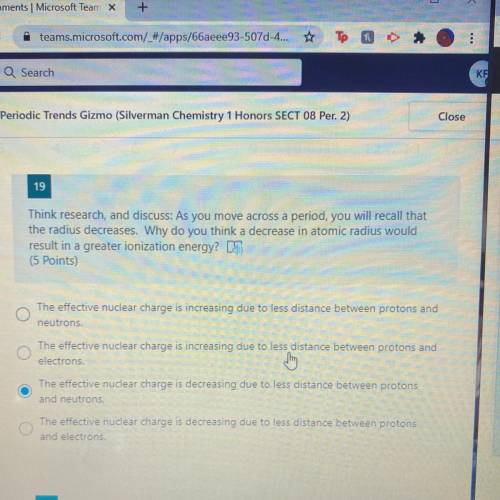
WILL GIBE BRAINLIEST Think research, and discuss: As you move across a period, you will recall that
the radius decreases. Why do you think a decrease in atomic radius would
result in a greater ionization energy?
(5 Points)
The effective nuclear charge is increasing due to less distance between protons and
neutrons.
O
The effective nuclear charge is increasing due to less distance between protons and
electrons.
The effective nuclear charge is decreasing due to less distance between protons
and neutrons.
The effective nuclear charge is decreasing due to less distance between protons
and electrons.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1


Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
WILL GIBE BRAINLIEST Think research, and discuss: As you move across a period, you will recall that...
Questions


Social Studies, 16.12.2020 09:30

Mathematics, 16.12.2020 09:30

Business, 16.12.2020 09:30

Mathematics, 16.12.2020 09:30


Biology, 16.12.2020 09:40


Geography, 16.12.2020 09:40


Social Studies, 16.12.2020 09:40


History, 16.12.2020 09:40

English, 16.12.2020 09:40



Mathematics, 16.12.2020 09:40

Chemistry, 16.12.2020 09:40


Mathematics, 16.12.2020 09:40



