Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 23.06.2019 09:10
In a 28 g serving of cheese curls there are 247mg of sodium. how much sodium is in a 12.5 ounce bag
Answers: 1

Chemistry, 23.06.2019 16:00
What is the consequence on your brain for snorting or taking amphetamines(meth/stimulant) as a 16 year old(minor)?
Answers: 2

Chemistry, 23.06.2019 19:10
Which us an example of the practical pursuit of alchemy? a. linking spiritual characteristics with material substances b. forming perfect substances c. developing metalworking technique d. transforming base metals
Answers: 2
You know the right answer?
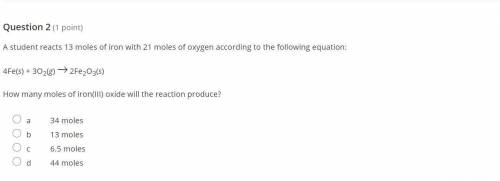
A student reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
4Fe(...
Questions


Biology, 19.03.2021 14:00


English, 19.03.2021 14:00

English, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00


History, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00

Chemistry, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00



Mathematics, 19.03.2021 14:00