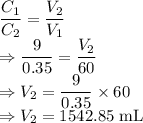Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
To what volume will you have to dilute 60.0 mL of a 9 M HCl solution to
make a 0.35 M HCl solution?...
Questions


Arts, 14.12.2020 02:20



Business, 14.12.2020 02:20

Mathematics, 14.12.2020 02:20


Health, 14.12.2020 02:20

Chemistry, 14.12.2020 02:20

English, 14.12.2020 02:20

History, 14.12.2020 02:20

Mathematics, 14.12.2020 02:20


Computers and Technology, 14.12.2020 02:20





Mathematics, 14.12.2020 02:20

Mathematics, 14.12.2020 02:20

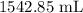
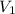 = Volume of HCl = 60 mL
= Volume of HCl = 60 mL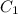 = Initial concentration of HCl = 9 M
= Initial concentration of HCl = 9 M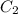 = Final concetration of HCl = 0.35 M
= Final concetration of HCl = 0.35 M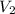 = Volume to be diluted
= Volume to be diluted