
Chemistry, 04.02.2021 14:00 bethanybowers4986
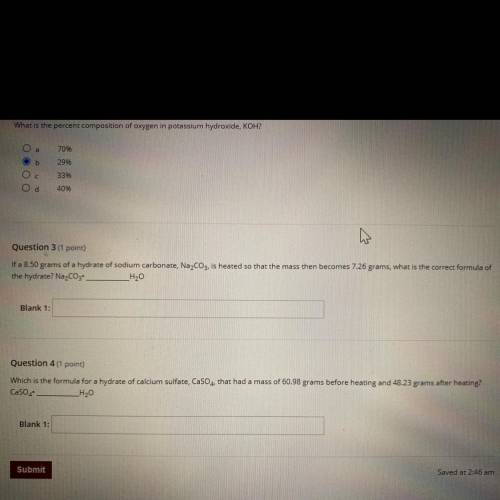
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that the mass then becomes 7.26 grams, what is the correct formula of
the hydrate? Na2CO3 _H20
Blank 1:
Question 4 (1 point)
Which is the formula for a hydrate of calcium sulfate, CaSO4, that had a mass of 60.98 grams before heating and 48.23 grams after heating?
CaSO4
_H20
Blank 1:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that th...
Questions


Mathematics, 01.03.2021 20:10

History, 01.03.2021 20:10

Mathematics, 01.03.2021 20:10




Mathematics, 01.03.2021 20:10



Mathematics, 01.03.2021 20:10


Health, 01.03.2021 20:10




Chemistry, 01.03.2021 20:10


History, 01.03.2021 20:10




