
Chemistry, 03.02.2021 03:50 lolyourenotpoppunk
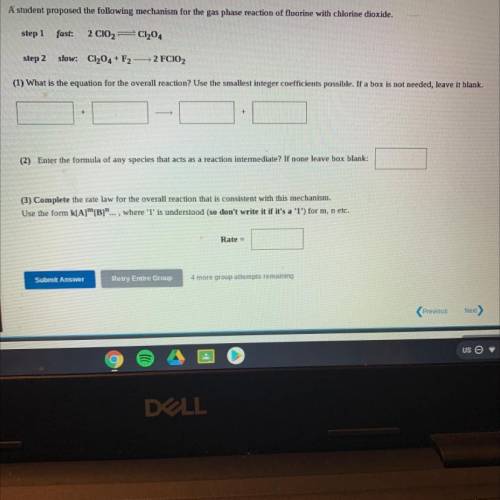
A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine dioxide.
step 1
fast:
2 C102=C1204
step 2
slow: C1204 + F2
2 FCIO2
(1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank.
+
+
(2) Enter the formula of any species that acts as a reaction intermediate? If none leave box blank:
(3) Complete the rate law for the overall reaction that is consistent with this mechanism.
Use the form k[A][B]"..., where 'l' is understood (so don't write it if it's a '1') for m, n etc.
Rate =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine diox...
Questions

Mathematics, 07.07.2019 16:30



Mathematics, 07.07.2019 16:30


Mathematics, 07.07.2019 16:30

Mathematics, 07.07.2019 16:30






Mathematics, 07.07.2019 16:30

Mathematics, 07.07.2019 16:30

Mathematics, 07.07.2019 16:30


English, 07.07.2019 16:30


History, 07.07.2019 16:30



