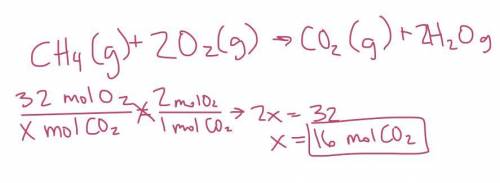Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

You know the right answer?
How many moles of carbon dioxide are formed when 32 moles of oxygen gas are consumed?
CH4(g) + 2O2(...
Questions

Social Studies, 04.03.2021 20:50

English, 04.03.2021 20:50



Mathematics, 04.03.2021 20:50

Mathematics, 04.03.2021 20:50




Social Studies, 04.03.2021 20:50

Business, 04.03.2021 20:50


Physics, 04.03.2021 20:50

Mathematics, 04.03.2021 20:50

Social Studies, 04.03.2021 20:50


English, 04.03.2021 20:50

Mathematics, 04.03.2021 20:50

Mathematics, 04.03.2021 20:50