
Chemistry, 02.02.2021 03:10 janellball16
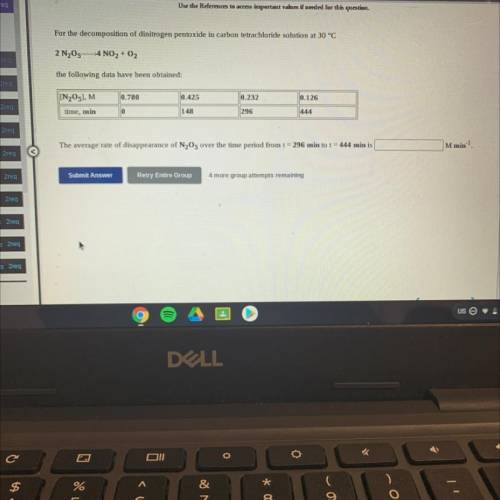
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO2 + O2
the following data have been obtained:
[N205], M
0.780
0.425
0.232
0.126
||444
time, min
0
148
296
M min-1
The average rate of disappearance of N2O5 over the time period from t = 296 min to t = 444 min is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N205-4 NO...
Questions

Mathematics, 18.04.2020 18:05


Mathematics, 18.04.2020 18:06


Chemistry, 18.04.2020 18:07



Mathematics, 18.04.2020 18:08




Social Studies, 18.04.2020 18:17

Mathematics, 18.04.2020 18:17

Social Studies, 18.04.2020 18:21








