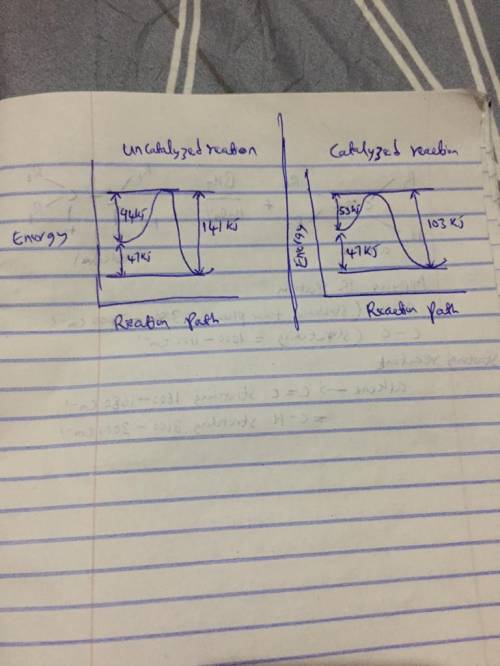
A catalyst decreases the activation energy of a particular exothermic reaction by 56 kJ/mol, to 35 kJ/mol. Assuming that the mechanism has only one step, and that the products are 78 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions. What is the activation energy for the uncatalyzed reverse reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
A catalyst decreases the activation energy of a particular exothermic reaction by 56 kJ/mol, to 35 k...
Questions

Mathematics, 10.11.2020 23:50


Physics, 10.11.2020 23:50




Mathematics, 10.11.2020 23:50

Mathematics, 10.11.2020 23:50



Mathematics, 10.11.2020 23:50


Mathematics, 10.11.2020 23:50




Advanced Placement (AP), 10.11.2020 23:50



Mathematics, 10.11.2020 23:50