Chemistry, 01.02.2021 22:40 ChasityN8491
A quantity of water is heated from 25.0°C to 36.4°C by absorbing 325 J of heat energy. If the specific heat of water is 4.18 J / g°C, what mass is this quantity of water?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
A quantity of water is heated from 25.0°C to 36.4°C by absorbing 325 J of heat energy. If the specif...
Questions

Mathematics, 31.03.2020 00:27


Chemistry, 31.03.2020 00:27

History, 31.03.2020 00:27

Mathematics, 31.03.2020 00:27















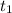 = 25 °C
= 25 °C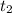 = 36,4 °C
= 36,4 °C )
)