Can someone with this-
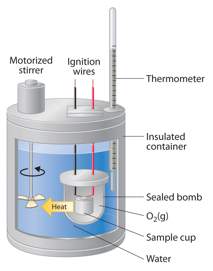
look at this picture. it shows a piece of apparatus manufactured to m...

Chemistry, 30.08.2019 03:30 tfyvcu5344
Can someone with this-
look at this picture. it shows a piece of apparatus manufactured to measure the energy released from food. it is called a calorimeter. the sample cup is where the food is placed.
explain how each of the following parts make the results more accurate.
a. ignition wires
b. stirrer
c. sealed “bomb”

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Questions



Social Studies, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

History, 12.12.2020 16:20


English, 12.12.2020 16:20

English, 12.12.2020 16:20






Mathematics, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20



