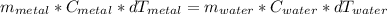Chemistry, 01.02.2021 09:20 mrtroll3289
A 13.9 - g piece of metal ( specific heat capacity is 0.449 /g^ C)who whose temperature is 54.2 degrees * C was added to a sample of water at 13.4 degrees * C in a constant - pressure calorimeter of negligible heat capacity . If the final temperature of the water is 15.6 °C, calculate the mass of the water in the calorimeter .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement about sound is not true? a. air particles travel with sound waves. b. sound waves cannot travel through a vacuum. c. sound waves exist even if no one hears them. d. air particles vibrate along the path of a sound wave.
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
A 13.9 - g piece of metal ( specific heat capacity is 0.449 /g^ C)who whose temperature is 54.2 degr...
Questions


History, 28.07.2019 20:00


Mathematics, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00

Physics, 28.07.2019 20:00








Social Studies, 28.07.2019 20:00

History, 28.07.2019 20:00

Social Studies, 28.07.2019 20:00


Physics, 28.07.2019 20:00

Mathematics, 28.07.2019 20:00