Chemistry, 31.01.2021 16:40 collinedwards5011
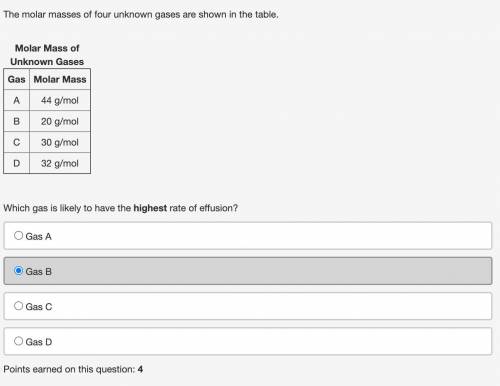
The molar masses of four unknown gases are shown in the table. Molar Mass of Unknown GasesGas Molar Mass
A 44 g/mol
B 20 g/mol
C 30 g/mol
D 32 g/mol
Which gas is likely to have the highest rate of effusion?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
The molar masses of four unknown gases are shown in the table. Molar Mass of Unknown GasesGas Molar...
Questions

Biology, 28.08.2019 05:40

History, 28.08.2019 05:40

Mathematics, 28.08.2019 05:40

Arts, 28.08.2019 05:40



Health, 28.08.2019 05:40

Social Studies, 28.08.2019 05:40











Biology, 28.08.2019 05:40

Social Studies, 28.08.2019 05:40