

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
An empty beaker and watch glass has a mass of 120.15g. Exactly 25.00 mL of an unknown liquid was add...
Questions


Mathematics, 16.07.2019 13:10

History, 16.07.2019 13:10

History, 16.07.2019 13:10



Mathematics, 16.07.2019 13:10

Mathematics, 16.07.2019 13:10


Chemistry, 16.07.2019 13:10


Mathematics, 16.07.2019 13:10



Chemistry, 16.07.2019 13:10

Mathematics, 16.07.2019 13:10

Biology, 16.07.2019 13:10


Geography, 16.07.2019 13:10

Computers and Technology, 16.07.2019 13:10

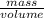
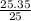 = 1.014g/mL
= 1.014g/mL 

