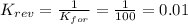I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure would
N2(g)+3H2(g)⇄2NH3(g)
a. increase [H2]
b. increase [NH3]
c. increase [N2]
d. have no effect
2. If K=100, then the value of K for the reverse reaction is
a. the same value
b. can only be determined by experimentation
c. the negative of the value for the forward reaction
d. 0.01

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
I don't understand how to solve.
1. For the exothermic reaction below, increasing the pressure woul...
Questions




History, 04.04.2020 05:57



Mathematics, 04.04.2020 05:57

History, 04.04.2020 05:57

Biology, 04.04.2020 05:57


Physics, 04.04.2020 05:57


Mathematics, 04.04.2020 05:57

English, 04.04.2020 05:57



Mathematics, 04.04.2020 05:57

Chemistry, 04.04.2020 05:57

Mathematics, 04.04.2020 05:57

Chemistry, 04.04.2020 05:57