Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1


Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
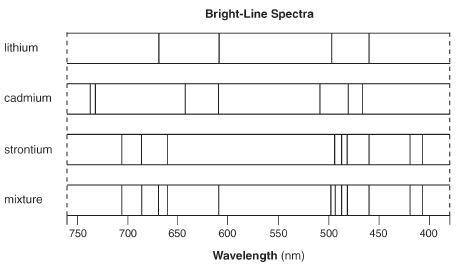
Answer the following question based on the bright line spectra. Explain, in terms of both electrons...
Questions

Biology, 20.02.2020 03:58

Geography, 20.02.2020 03:58


Social Studies, 20.02.2020 03:58


Chemistry, 20.02.2020 03:58




History, 20.02.2020 03:58

Mathematics, 20.02.2020 03:58

Mathematics, 20.02.2020 03:58