Chemistry, 29.01.2021 16:40 gracedaniels68
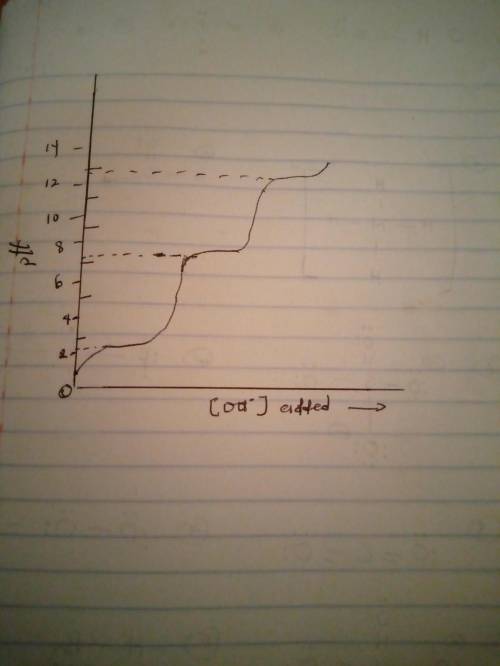
Phosphoric acid (H3PO4) can give up three protons, each with different pKa values consisting of 2.12, 7.21, and 12.67. If plotting pH as a function of sodium hydroxide solution added, which titration curve will be formed?

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
If this equation was completed which statement would it best support
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Phosphoric acid (H3PO4) can give up three protons, each with different pKa values consisting of 2.12...
Questions

Mathematics, 24.09.2019 16:00



Health, 24.09.2019 16:00





Biology, 24.09.2019 16:00




Social Studies, 24.09.2019 16:00

Mathematics, 24.09.2019 16:00

English, 24.09.2019 16:00

Physics, 24.09.2019 16:00

English, 24.09.2019 16:00

History, 24.09.2019 16:00

Mathematics, 24.09.2019 16:00

Social Studies, 24.09.2019 16:00