
Chemistry, 29.01.2021 14:00 kaylakaye9840
50POINTS!
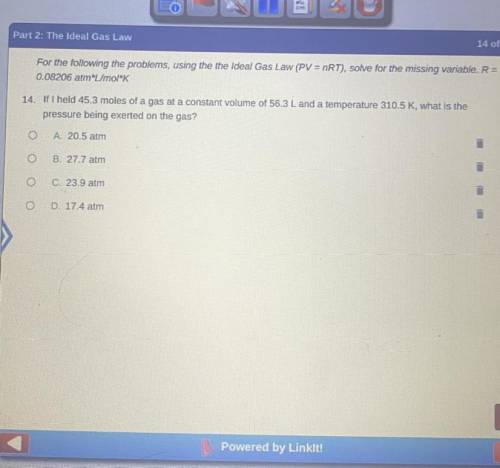
If I held a 45.3 moles of a gas at a constant volume of 56.4L and a temperature 310.5k, what is the pressure being exerted on the gas?
A. 20.5 atm
B. 27.7 atm
C. 23.9 atm
D. 17.4 atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
50POINTS!
If I held a 45.3 moles of a gas at a constant volume of 56.4L and a temperature 310.5k, w...
Questions



Mathematics, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10

English, 20.05.2021 19:10

Mathematics, 20.05.2021 19:10


Mathematics, 20.05.2021 19:10

History, 20.05.2021 19:10








Mathematics, 20.05.2021 19:10



