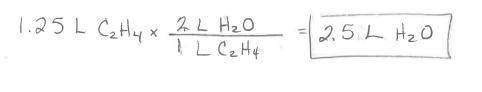Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1

Chemistry, 23.06.2019 11:20
Sandy is building a small toy car. he wants to use a balloon to power the toy car. he fills a balloon with air and then attaches a straw to the balloon. he tapes the balloon-straw combination to the car and then releases the end of the balloon. the toy moves forward as the air from the balloon comes out the back of the straw. what can sandy do to make the toy car move faster? a) use less air in the balloon. b) blow up the balloon more. c) use a longer straw. d) use larger tires.
Answers: 2
You know the right answer?
Ethylene burns in oxygen to form carbon dioxide and water vapor:
C2H4(g) + 3 02(g) --> 2 CO2(g)...
Questions



Mathematics, 30.06.2019 16:30


Mathematics, 30.06.2019 16:30


Mathematics, 30.06.2019 16:30

Mathematics, 30.06.2019 16:30


English, 30.06.2019 16:30







English, 30.06.2019 16:30



English, 30.06.2019 16:30