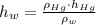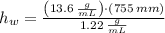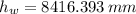Chemistry, 28.01.2021 22:20 Hazeleyes13
Suppose you were to construct a barometer using a fluid with a density of 1.22 g/mL. How high would the liquid level be in this barometer if the atmospheric pressure was 755 torr? (Mercury has a density of 13.6 g/mL.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

You know the right answer?
Suppose you were to construct a barometer using a fluid with a density of 1.22 g/mL. How high would...
Questions


History, 19.03.2020 21:51





Biology, 19.03.2020 21:52

Mathematics, 19.03.2020 21:52


English, 19.03.2020 21:52










 ), measured in grams per mililiter, and height of fluid (
), measured in grams per mililiter, and height of fluid (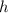 ), measured in milimeters. Two barometers with distinct fluids are equivalent when both have the same hydrostatic pressure. Then, we construct the following relationship:
), measured in milimeters. Two barometers with distinct fluids are equivalent when both have the same hydrostatic pressure. Then, we construct the following relationship: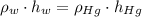 (1)
(1)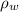 ,
, 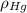 - Densities of fluid and mercury, measured in grams per mililiter.
- Densities of fluid and mercury, measured in grams per mililiter.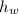 ,
,  - Heights of fluid and mercury columns, measured in milimeters.
- Heights of fluid and mercury columns, measured in milimeters.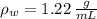 ,
, 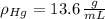 and
and 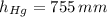 , then the liquid level of this barometer is:
, then the liquid level of this barometer is: