FILL OUT THE GRAPH AND ANSWER THE QUESTIONS WILL MARK BRAINLIEST
PIC OF GRAPH ATTACHED
...

FILL OUT THE GRAPH AND ANSWER THE QUESTIONS WILL MARK BRAINLIEST
PIC OF GRAPH ATTACHED
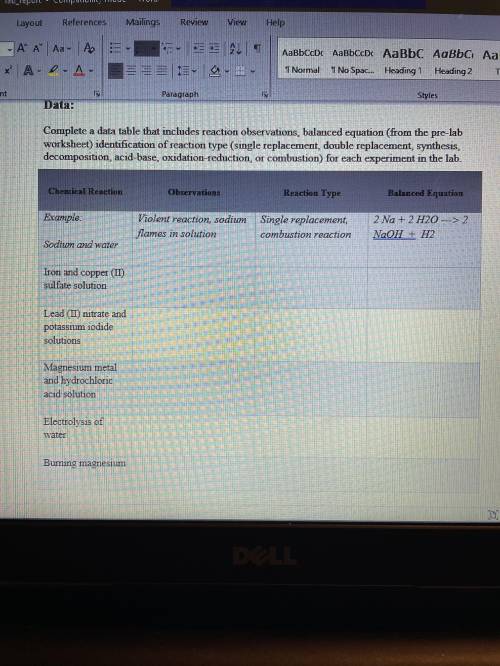
Conclusion:
Write a conclusion statement that addresses the following questions:
• Summarize your observations of each reaction. Based on the products made, were your predictions of chemical reaction type correct?
• Some of the reactions you conducted can be categorized as more than one type of reaction. Which reactions are these, and what are the types of reactions?
• How do you think the investigation can be explored further?
Post-Lab Reflection Questions
Answer the reflection questions using what you have learned from the lesson and your experimental data. It will be helpful to refer to your chemistry journal notes. Answer questions in complete sentences.
1. If you were to measure the mass of magnesium and hydrochloric acid before combining them in the test tube, how would that mass compare to the mass of reactants left in the test tube after the reaction? Explain your answer and how it corresponds to the law of conservation of mass.
2. In what other ways could you test your predictions to confirm their accuracy?
3. How do the chemical reactions in this lab activity compare to nuclear reactions, such as fission and fusion?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Your answer should have the same number or significant figures as a he starting measurement. 3201 ml =
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
Questions









World Languages, 06.07.2019 21:20






History, 06.07.2019 21:20







